WEEL Research
We are conducting intensive research to remove the pollutants from water, including Heavy metals such as Arsenic and Chromium, radioactive elements for an example cesium and strontium, recovery of nutrients from wastewater such as phosphorus and nitrate and Antibiotics such as ciprofloxacin and Chloramphenicol. In addition to the environmental remediation, we are also working on renewable bioenergy development especially methane and hydrogen production by anaerobic digestion system and electricity generation by microbial fuel cells. We are using the advantages and the benefits of nanotechnology to clean our environment, especially water treatment, resources recovery and bioenergy production from waste, in this regard, we are synthesizing various types of noble nanomaterials which have an extremely small size, high surface area to volume ratio and high surface reactivity compare with bulk materials.
Research Activities
Adsorption and Degradation of Emerging Pharmaceuticals and Personal Care Products from Contaminated Water Bodies via the Application of Iron-Based Nanoparticles and Nanocomposites
Pharmaceuticals and personal care products (PPCPs) are groups of organic compounds with distinctive chemical and physical characteristics that are originated to protect human lives, improve the daily life of individuals, and help farmers to maintain and enhance their agricultural production The production and consumption of PPCPs are dramatically increasing globally over the past years because of their significant benefits in both the medical and the economical fields. However, the excessive applications of these chemicals in human and animal medicine, resulted in frequently detection of hundreds of PPCPs, universally, in many surface waters and groundwater with concentrations ranging from ng/L to µg/L. The continual presence of PPCPs in our water resources is alarming and raising the red flag as they are biologically active compounds which may put the lives of human and animals under serious risk by promoting lethal diseases in water. The main driving forces to conduct such research are to protect the human and animals from receiving contaminated drinking water by PPCPs and prevent any possible future pandemics that may results from ignoring the existence of these chemicals in our drinking water resources.
Improving the Characteristics of Iron Nanoparticles via Non-Magnetic/Eco-Friendly Coatings
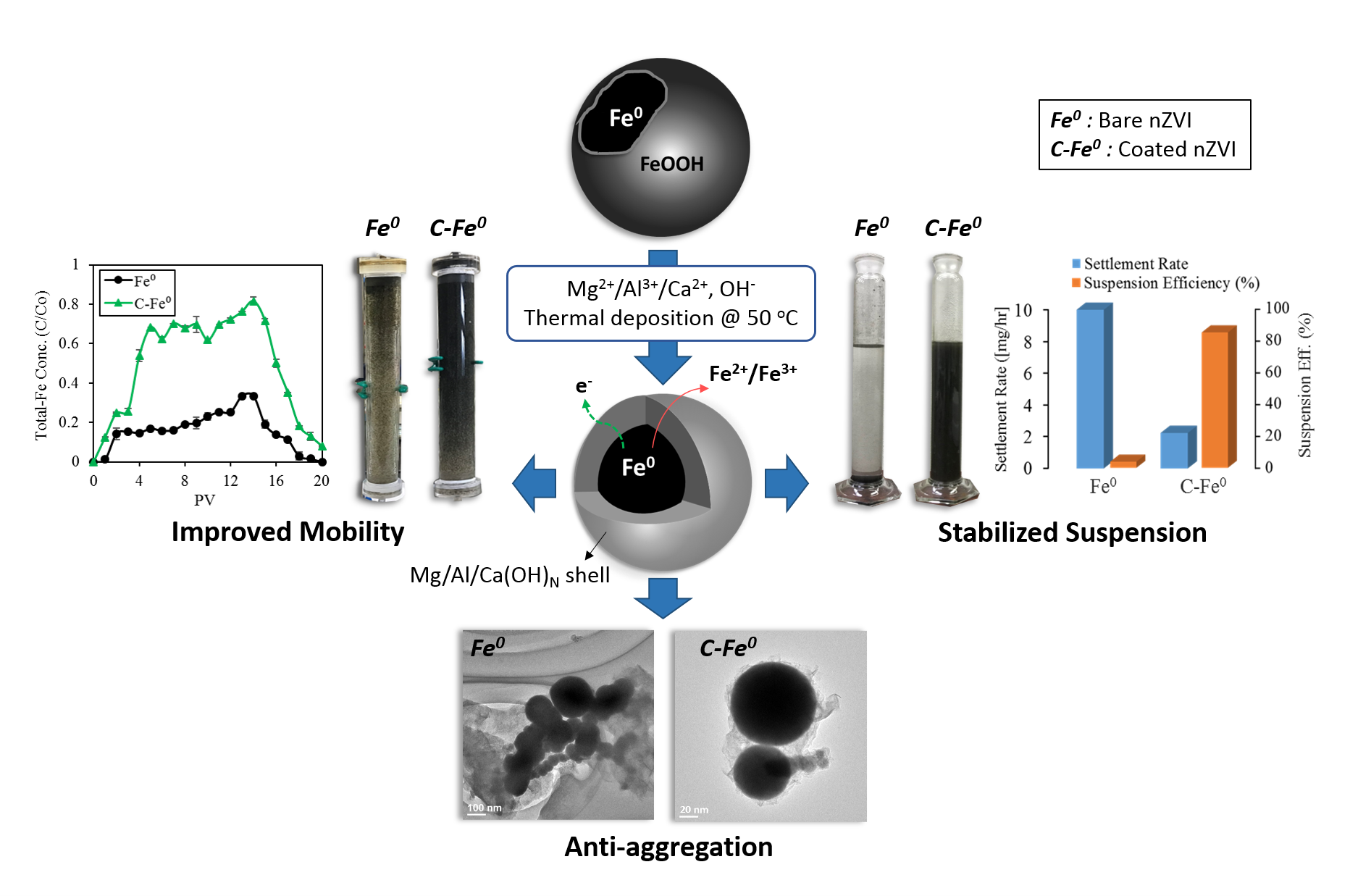
Recently, iron nanoparticles have gained special attention in water treatment owing to its superior features such as possessing diverse mechanisms of contaminant removal, high reducing potential, and the relatively low cost. However, bare Fe0 particles that prepared without any support tend to aggregate together, thereby losing their reactivity, stability, and soil deliverability. Therefore, the search for methods to improve the important aspects of iron nanoparticles are unstoppable attempts. Hence, this research focuses on coating iron nanoparticles with different layered hydroxide coatings (magnesium, aluminum, and calcium hydroxides) to enhance their aqueous suspension stability and transportability within the porous media. The presented coated iron nanoparticles could be promising towards enhanced performance of the reactive nanoparticles in real water treatment applications.
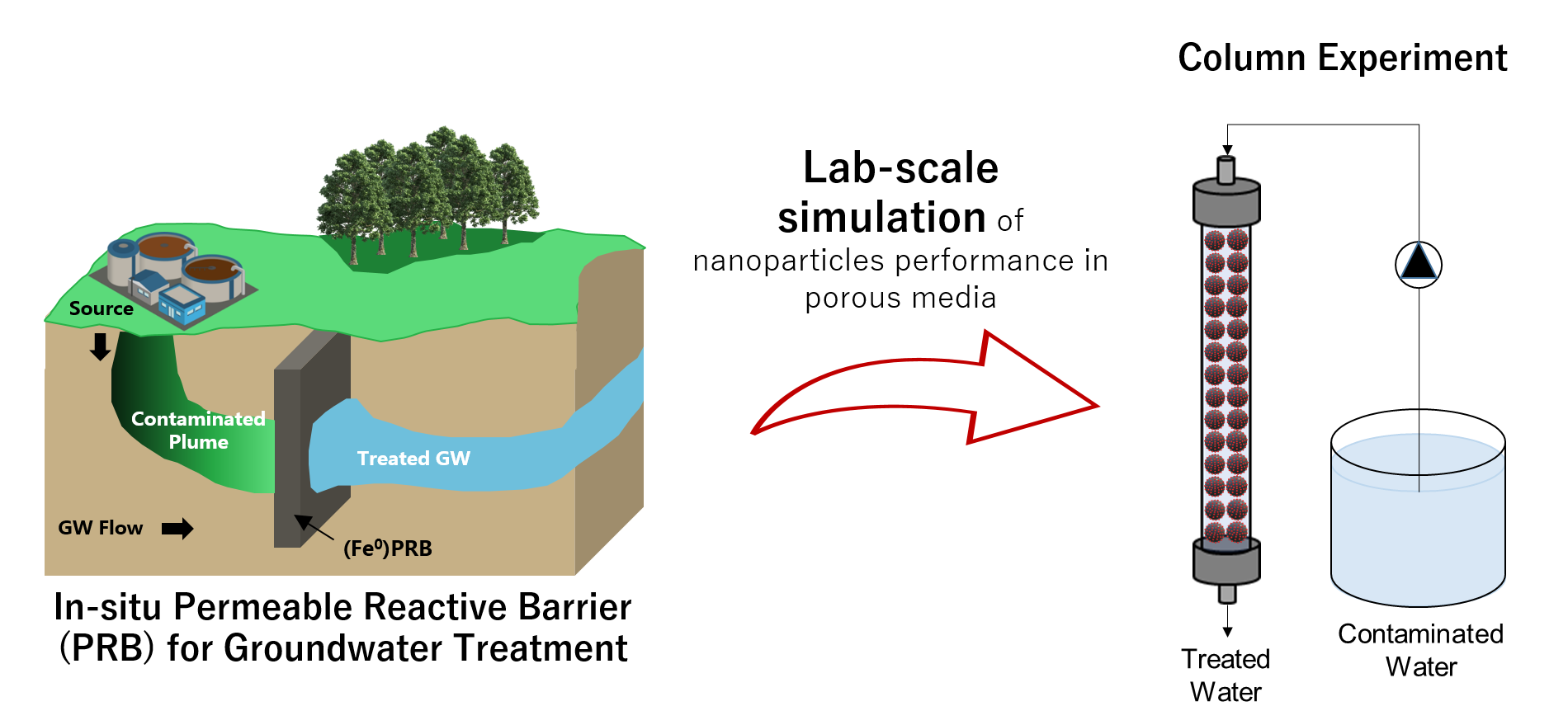
Reactive Solute Transport Modeling of Groundwater Contaminants Species in Porous Media
Reactive transport modeling in porous media refers to the creation of computer models integrating chemical reaction with transport of groundwater through the aquifers. Such models predict the distribution in space and time of the chemical reactions that occur along a flow path. Reactive transport modeling are often relied upon to predict the migration of contaminant plumes; the mobility of radionuclides in waste repositories; and the biodegradation of chemicals in landfills. When applied to the study of contaminants in the environments, they are known as fate and transport models. Numerical simulation is considered to be a significant approach that can be used to predict the performance of groundwater remediation techniques such as permeable reactive barrier (PRB). Owing to the difficulty of obtaining a real field data of PRBs long-term studies, conducting laboratory-scale column experiments is necessary for preliminary design of such field applications. Consequently, integrating experiment-based developed models in PRBs design is an effective way to predict its performance through the groundwater flow.
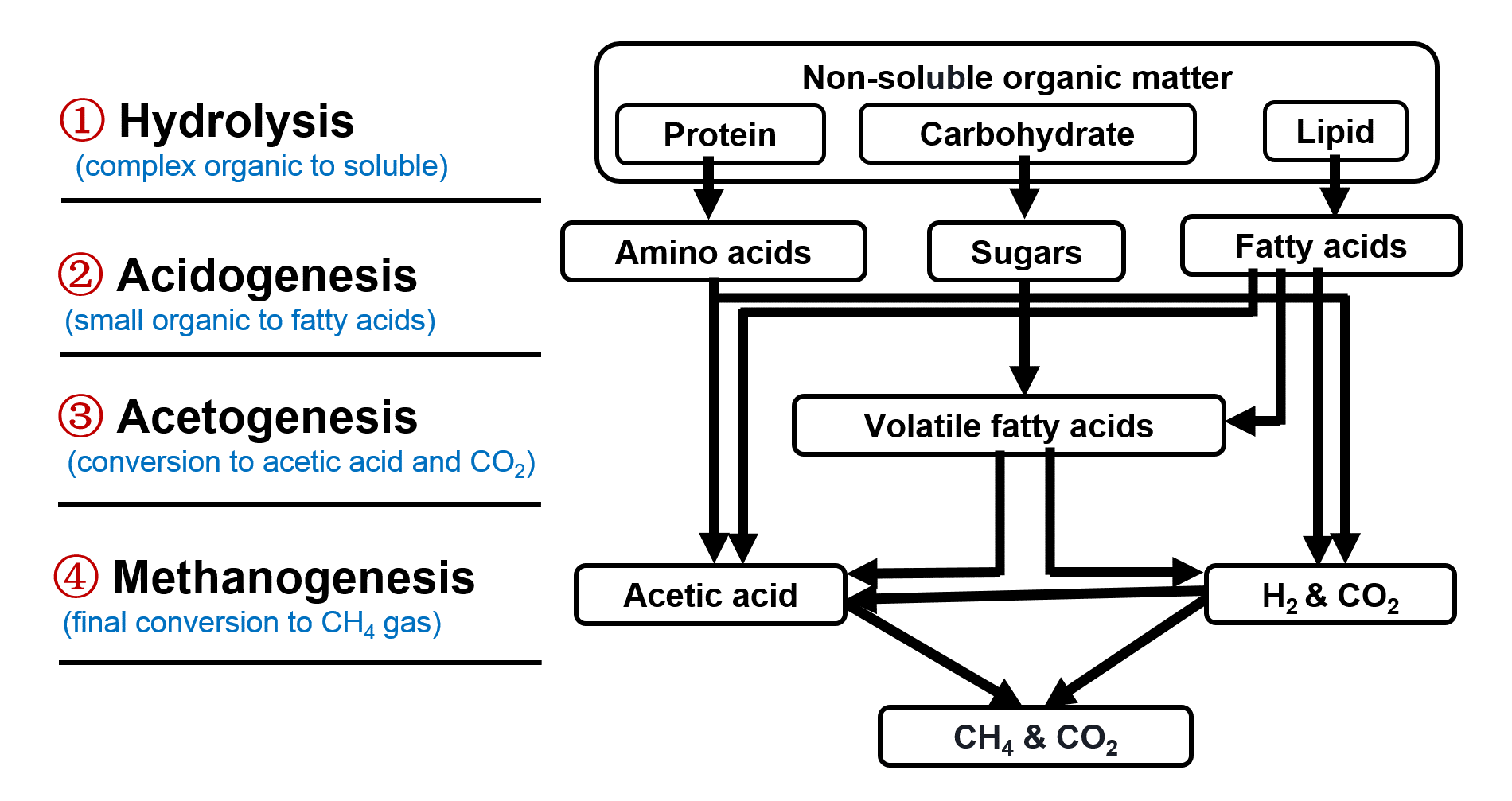
Biogas Generation from Wastewater Sludge via Anaerobic Digestion Systems
Anaerobic digestion is an established technology for the treatment of wastes and wastewater. The final product is biogas: a mixture of methane (55-75 vol%) and carbon dioxide (25-45 vol%) that can be used for heating, upgrading to natural gas quality or co-generation of electricity and heat. The anaerobic digestion process can be subdivided into the shown four phases, each requiring its own characteristic group of micro-organisms.
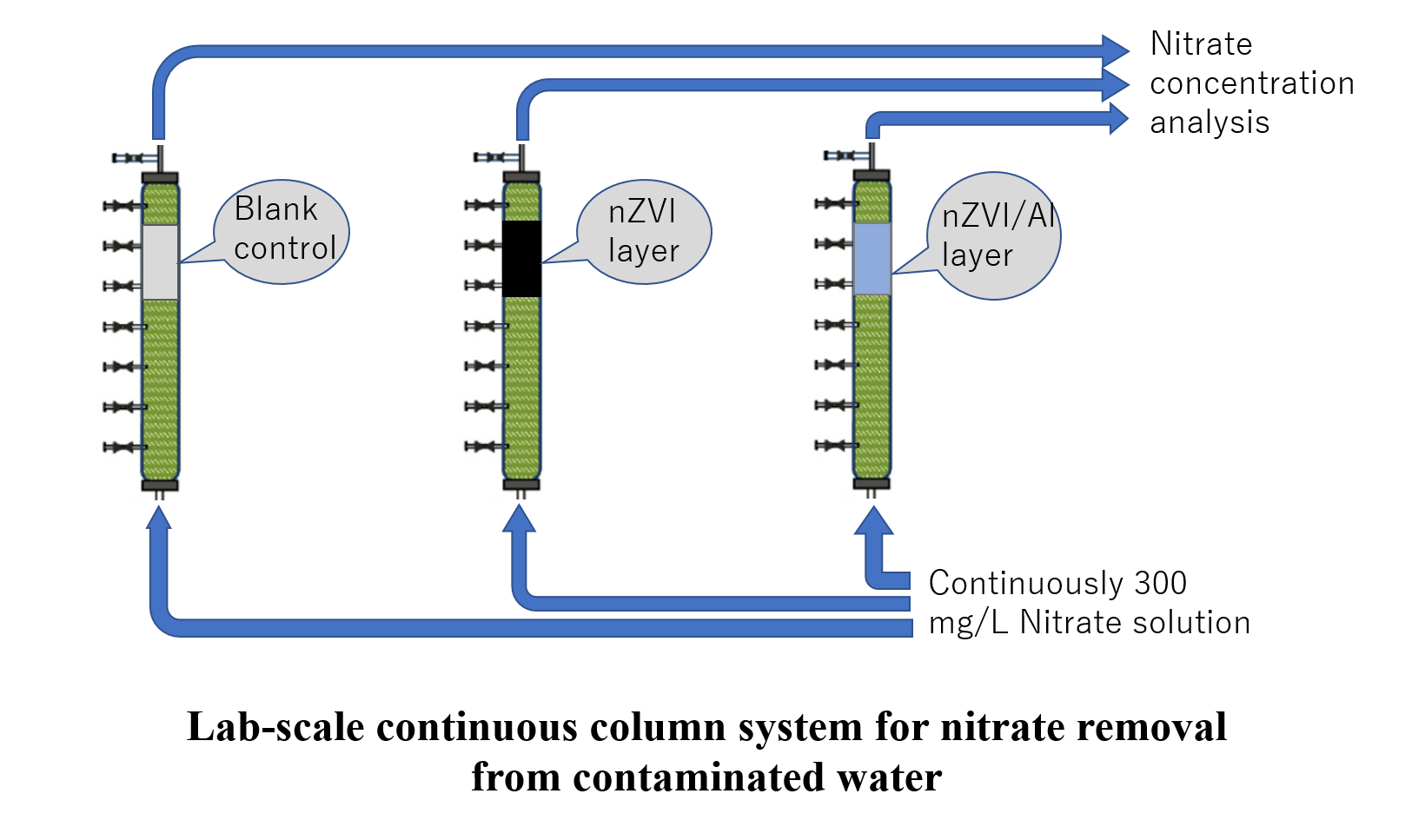
Zero Valent Al/Fe Nanoparticles Synthesis for Efficient Removal of Nitrate in a Lab-Scale Continuous Column System
Nitrate is a common contaminant in water bodies, how to efficiently remove it has always been a hot topic. At present, the commonly used method is to reduce nitrate in water by nano-zero-valent iron (nZVI). This method is very efficient and has very little secondary pollution. However, due to oxidation, the lifetime of nZVI has always been a problem in practical applications. In this experiment, the aluminum metal element and nZVI are combined as zero valent Al/Fe nanoparticles. The Fe3+ , Fe2+ ions are reduced to Fe0 by the electrons released by the aluminum metal element, which increases the lifetime of the nano-zero-valent iron (nZVI) and prolongs the reactive performance time. It is also practiced in the column system to develop a lab-scale continuous column system verifies the nitrate removal efficiency of this material under long-term conditions.
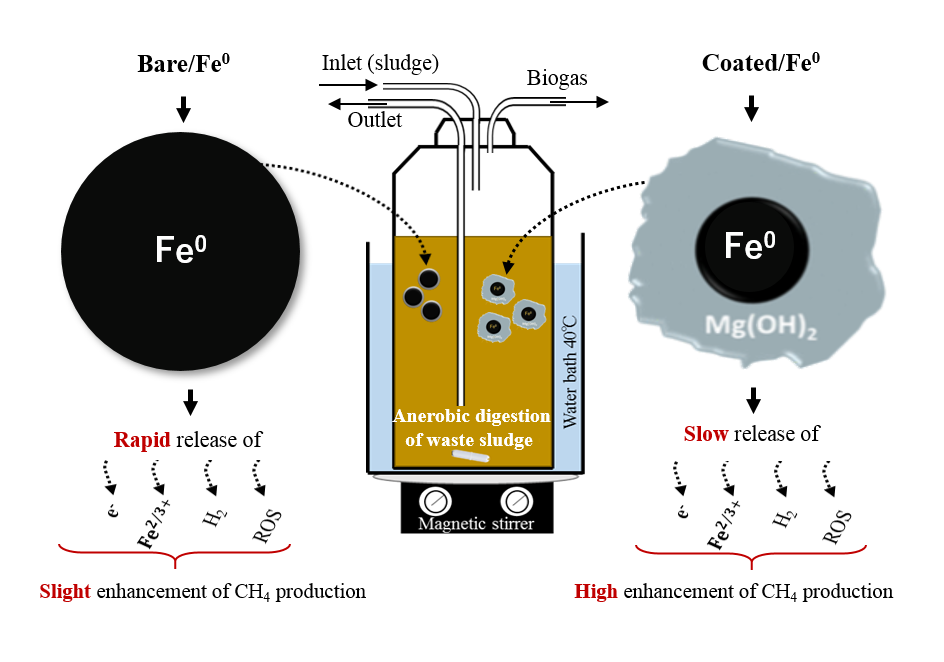
Integration Coated Iron Nanoparticles in Anaerobic Digestion Process for Efficient Conversion of Organic Waste into Methane Gas
There are different strategies to overcome the low conversion of organic waste into methane gas during the anaerobic digestion process, including bio-augmentation, nano additives (Fe0 & Coated (C/Fe0)), co-digestion, and pre-treatment of waste. So, in this study magnesium hydroxide was considered for the coating modification of Fe0 as the nano additive into the anerobic digestion of wastewater sludge towards an efficient methane generation. The bare/Fe0 nanoparticles were synthesized following the chemical reduction reaction and coated with Mg(OH)2 following the thermal deposition method. Experiments were conducted using two experimental set-ups: batch and long-term continuous operation. Batch tests were performed using different dosages of Fe0 or C/Fe0, while the continuous operation conducted using the optimum dosage of C/Fe0 obtained from batch investigations. Results showed that methane production improved by 4.7% at the optimum dosage of Fe0.The slight positive effect is attributed to the fast corrosion of Fe0. While methane production improved by 46.6% using the C/Fe0 at the optimum dose (25 mg/gVS) and optimum coating ratio (Mg/Fe0:0.5). In the continuous operation, methane production improved by 120 % with the addition of C/Fe0 at the optimum dosage (25 mg/gVS) and optimum coating ration (Mg/Fe0:0.5). Moreover, biogas methane content was enhanced by the addition of C/Fe0, which would decrease the cost of biogas upgrading steps.
Treatment of Emerging Contaminant (Pharmaceuticals) from Water using NZVI/Graphene TiO2 Nanowire Composite
Pharmaceuticals are an example of emerging organic contaminants, which have a high potential for wreaking havoc on the environment and ecosystems. In addition, this emerging contaminant could also have harmful consequences on human health because of its presence in ground and surface waters and could infinitely contaminate drinking water. Therefore, the primary goal of this research is to work with graphene oxide and its potential composite to remove this emerging contaminant from waste and water sources. Water treatment using graphene oxide incorporating other nanomaterials like zero-valent iron and titanium oxide is expected to offer great effectiveness in contaminants removal. Nanomaterials will be used as incorporated prospective materials in this application due to their numerous advantages. The study of nanocomposites for nano-adsorbents has seemingly become trendy due to their versatility in removing harmful pollutants such as pharmaceuticals from water or aqueous solutions. The synthesis method of graphene oxide incorporated with other nanomaterials will be optimized to remove emerging contaminants through this study. Furthermore, the ideal water treatment parameters for the produced composite application regarding contaminant removal efficiency will be examined.
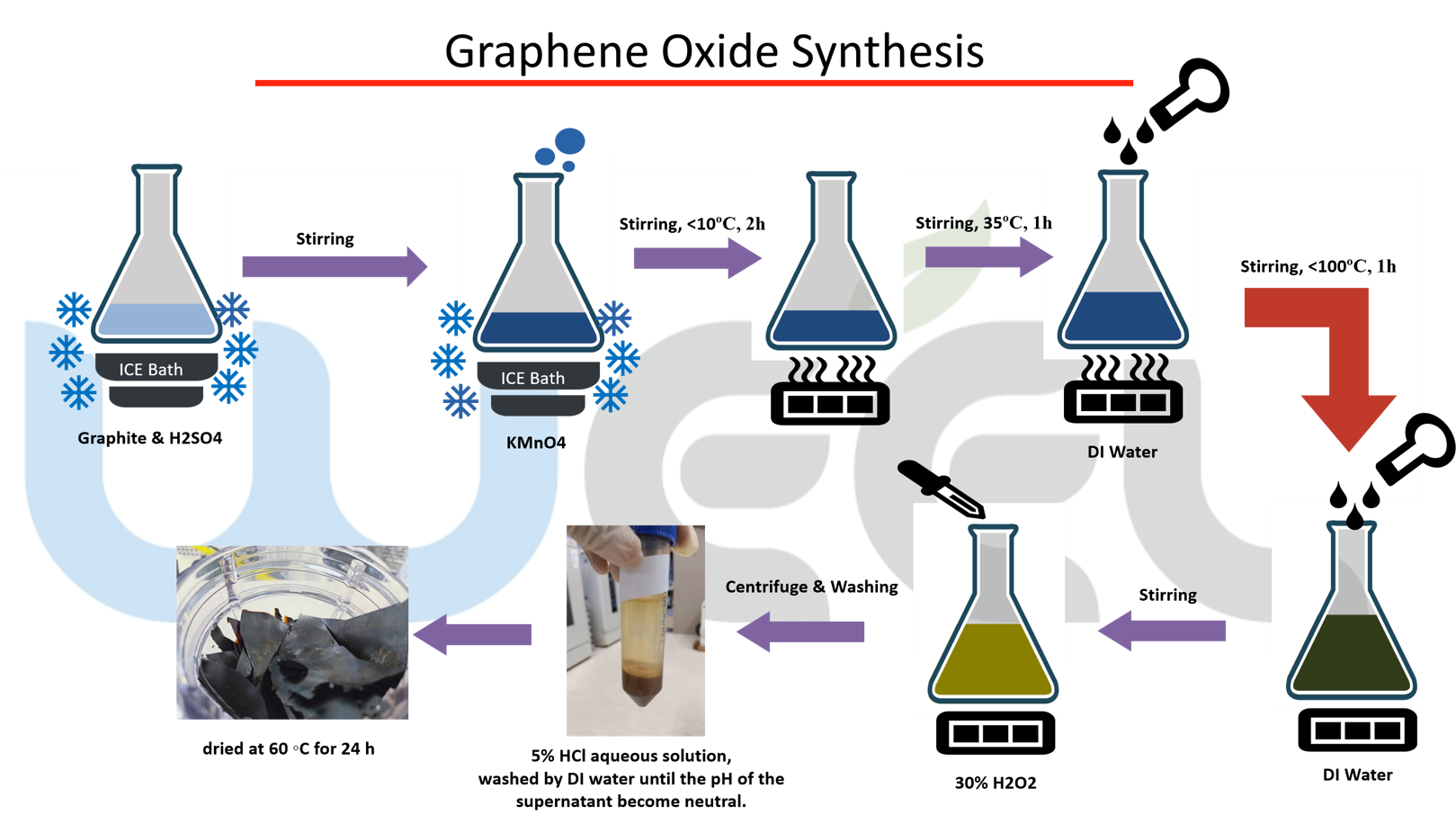
Integrating Titanium Oxide Nanowires Supported Nanoscale Zero-Valent Iron in Microbial Fuel Cells (MFCs) for Electricity Bio-Generation
Wastewater treatment is an expensive and energy-intensive process. In this regard, biological wastewater treatments like Microbial fuel cells (MFCs) is an effective treatment system for Organic matter removal and electricity production, but there are challenges that face this system, and limit the power density of it, one of them is the poor extracellular electron transfer, which can be improved by using Titanium nanowires, which will be a carrier in electrons transfer between bacteria and Anode electrode to enhance electricity. Combining nanoscale zero-valent iron with titanium nanowires can amplify the electricity generation and pollutants elimination.
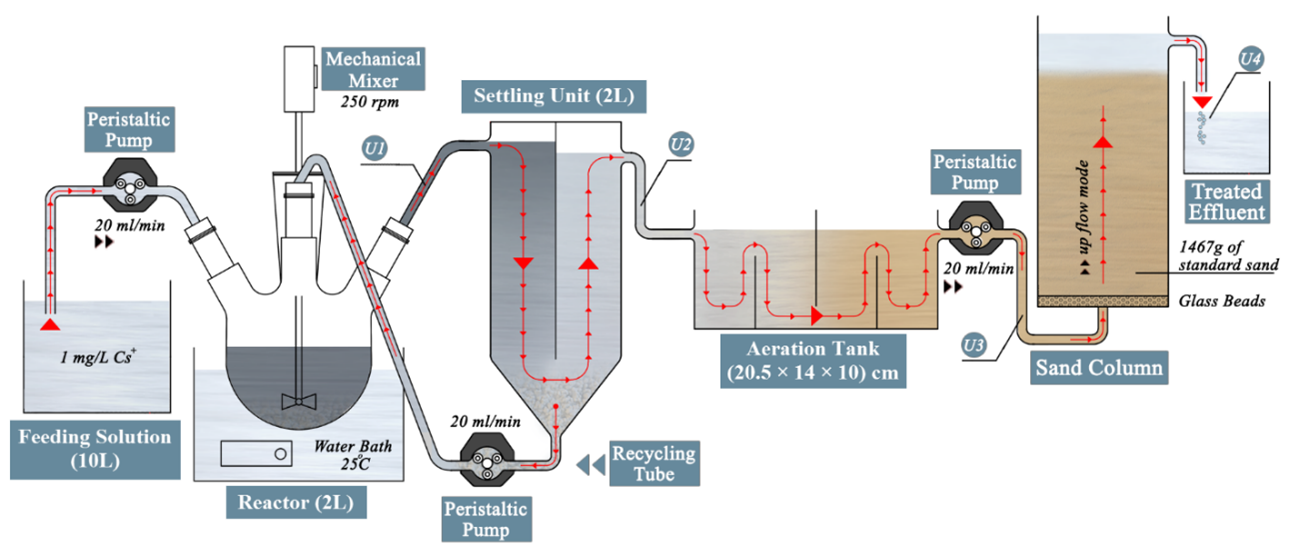
Magnetic Zeolite Synthesis for Efficient Removal of Cesium in a Lab-Scale Continuous Treatment System
Radioactive cesium was resealed to the environment as a result of many nuclear incidents. An effective treatment system is urgently needed to safely handle radioactive cesium-contaminated waters. Based on nanoscale zerovalent iron (nZVI) and zeolite, nine adsorbents were synthesized and applied to remove cesium from aqueous solutions. Magnetic zeolite composite (Ze/Fe0) was selected as the ideal adsorbent for treating cesium contaminated waters in a lab-scale continuous treatment system (LSCTS). The optimization process of the (Ze/Fe0) composite revealed that 1:1 is the optimum mass ratio between zeolite and nZVI. Furthermore, the optimization process proved that the initial pH and temperature have no significant effect on the adsorption of cesium by (Ze/Fe0) composite and the optimum dosage of (Ze/Fe0) composite is 5 g L−1. LSCTS succeed to treat continuous flows of 1 mg L−1 cesium contaminated water with 100% overall removal efficiency.
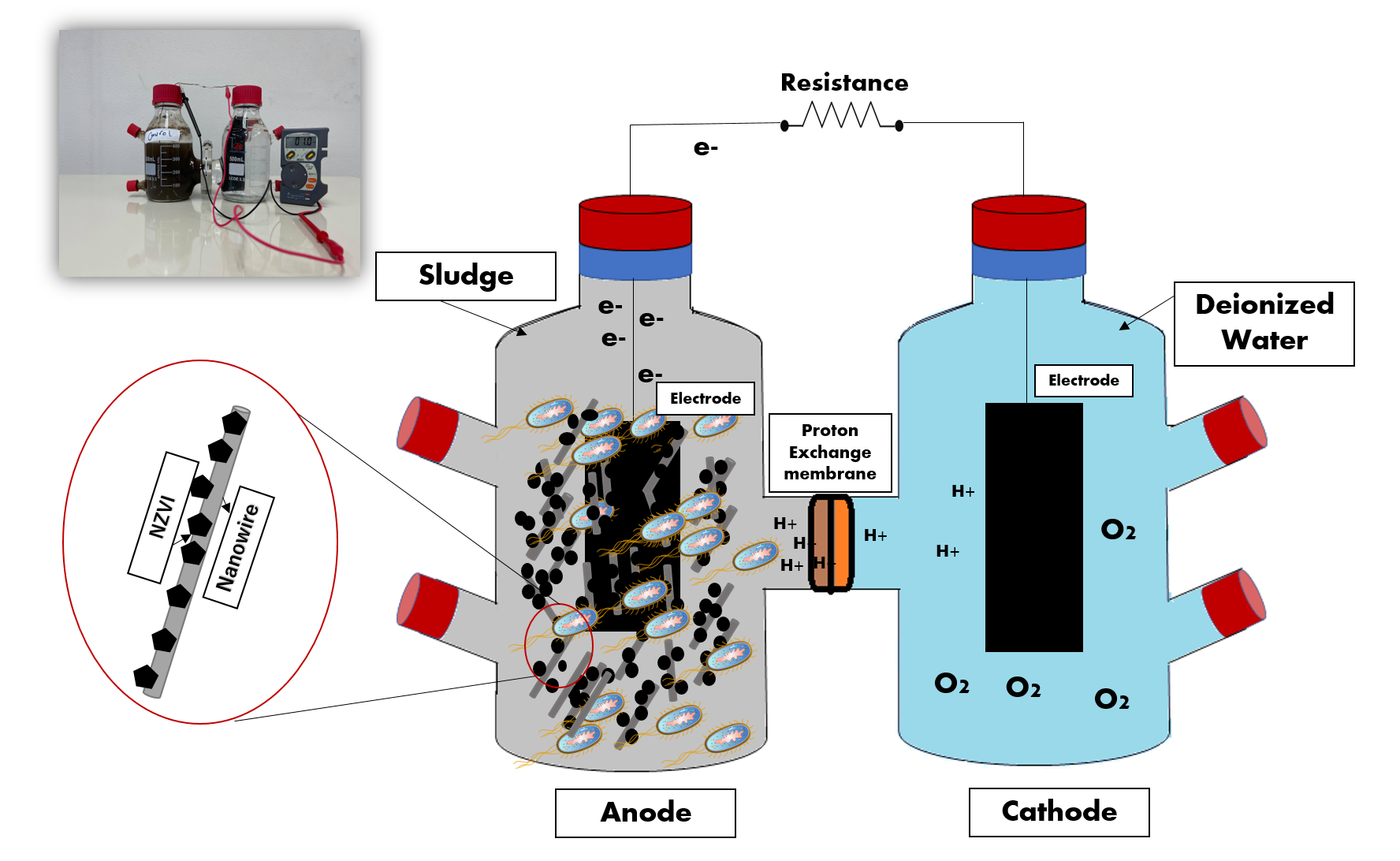
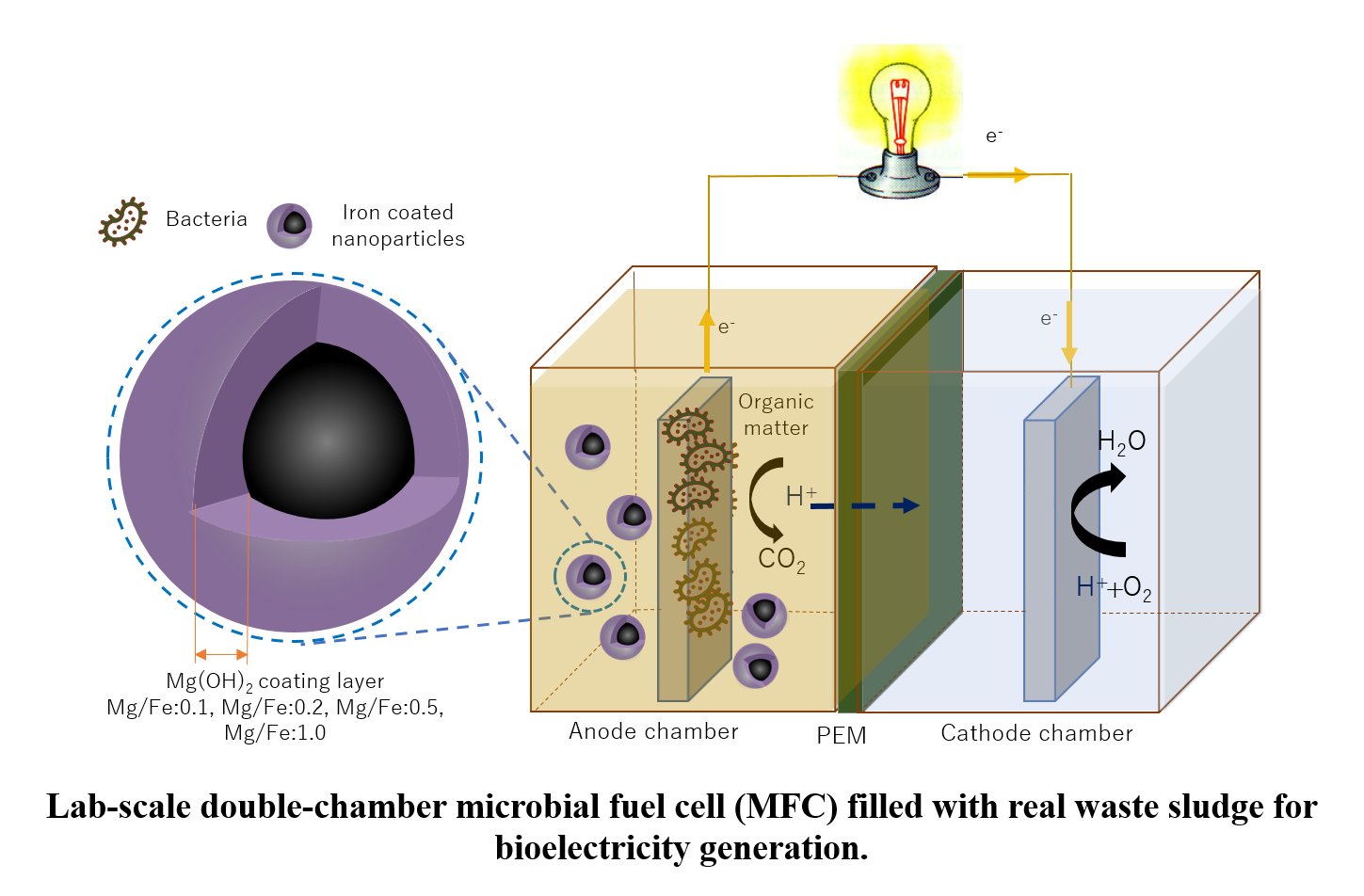
Power Generation in Microbial Fuel Cells (MFCs) from Real Sludge Using Fe@Mg(OH)2 Nanoparticles
Microbial fuel cells (MFCs) have drawn attention for many years as a versatile technique for eco-friendly energy production, waste management, and bioremediation. Hence, this research work examines the use of bare and coated Fe0 nanoparticles with Mg(OH)2 in a lab-scale doble-chamber MFC. Four different Mg/Fe coating ratios were separately added to the anode chamber and comparatively evaluated for power generation. The study examined four waste sludges, different pH, and aerobic enriched cathode chambers' effect on current production. Results showed a promising potential in the voltage output with more than 4 times increase comparing with the control, in addition to a maximal power density of 1387.25 µW/g.VS; 3.204 mA. The current generation's stability was achieved under neutral pH (158.48 µW/g.VS; 0.932 mA), and the power density output decreased by 82.80 % under aerobic conditions. The findings of this work suggests that bacterial multiplication is not the only decisive parameter in MFC's output, and additional parameters should be investigated.
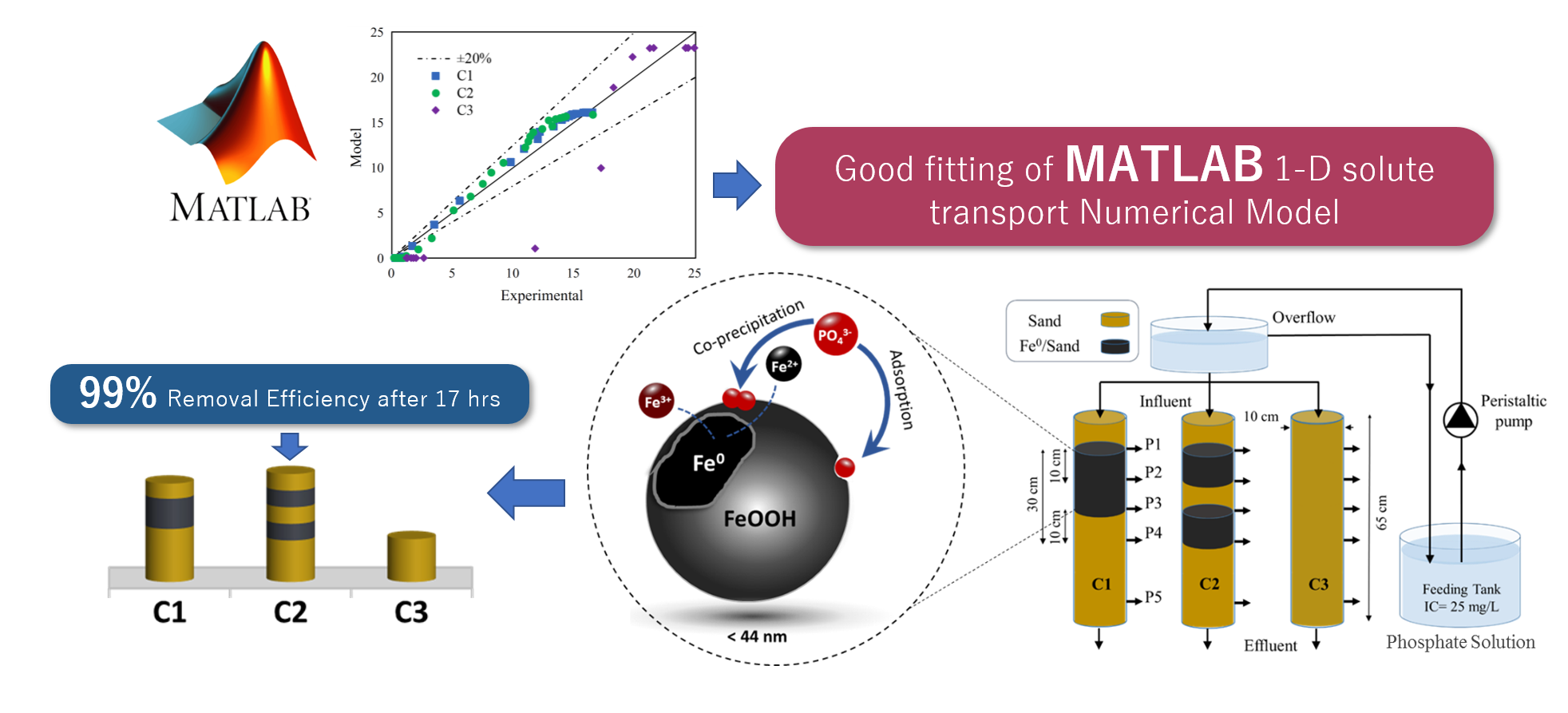
Phosphate Removal through Nano-Zero-Valent Iron Permeable Reactive Barrier; Column Experiment and Reactive Solute Transport Modeling
This research work investigated the efficacy of nano-zero-valent iron (nZVI) permeable reactive barriers (PRBs) as an in-situ phosphate removal method. Batch equilibrium experiments were conducted to determine maximum adsorption capacity of nano-iron particles for phosphate to be 54.34 mg-P/g-Fe. Short-term experiment was performed for a period of a month through three sandy soil columns with different configurations of nZVI reactive layers. Initial concentration of 25 (PO4–P) mg/L was introduced to the columns, while effluent samples were collected for analysis. Numerical model was developed to simulate the 1-D advective–dispersive reactive transport of phosphate through the porous media in the three columns. Sensitivity analysis of model parameters was performed, expressed by the change in the effluent concentration with respect to variation in the value of model critical parameters. Optimization of transport process in columns was based on minimizing the sum of squared error values between measured and predicted effluent concentration. Phosphate breakthrough curves implied that the two reactive layers showed the best performance in phosphate removal with a maximum efficiency of 98.9% after only 17 h from the beginning of the experiment. The model verification with experimental data showed a reasonable agreement with a correlation coefficient (R2) ranging from 0.97 to 0.99. The results in this study confirmed that such presented model can be used for the promotion of the preliminary design of PRBs.
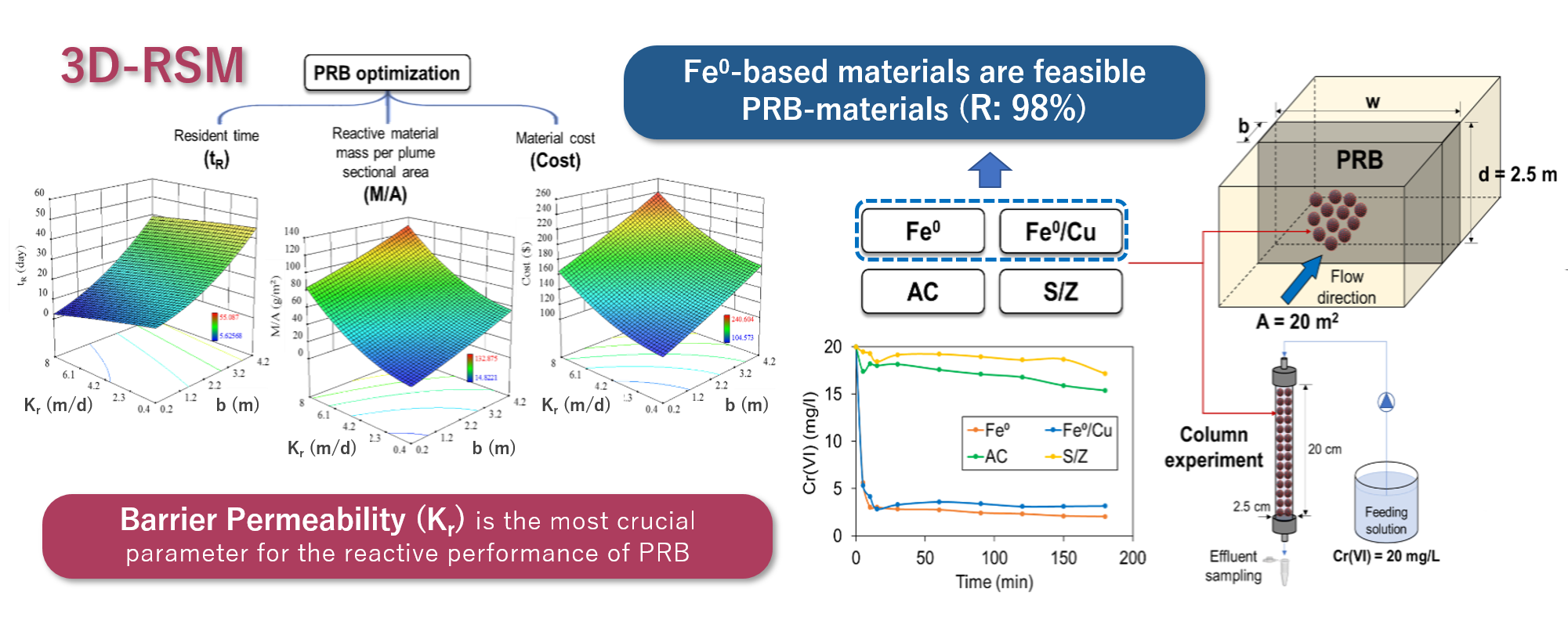
Multi-Objective Optimization of Permeable Reactive Barrier Design for Cr(VI) Removal from Groundwater
This research work aimed to develop a practical approach for the optimal permeable reactive barrier (PRB) design towards Cr(VI) removal from groundwater. Batch and column experiments were performed to investigate the characteristics of the four proposed reactive materials; nanoscale zero-valent iron (Fe0), bimetallic nanoscale zero-valent iron (Fe0/Cu), activated carbon (AC) and sand/zeolite mixture (S/Z). Moreover, the Response Surface Methodology (RSM) was considered for optimizing the design variables of the PRB based on the practical factorial analysis. Results revealed that Fe0 and Fe0/Cu showed high performance in Cr(VI) removal, with a slight superiority to Fe0, with final removal efficiency values of 89.7 and 84.1%, respectively. ANOVA statistical analysis revealed that quadratic polynomial model was the best model, corresponding to the highest correlation efficiency and adequate precision, to describe the relationships in the four PRB's cases between the selected dependent variables; resident time (tR), reactive material mass per sectional area of contaminant plume (M/A) and reactive material cost (Cost) towards the independent parameters; barrier thickness (b) and permeability (Kr). RSM-optimization revealed that Fe0 is the most feasible reactive material, comparing to the other considered materials, with respect to the optimal conditions regarding the long residency (tR = 22 days) and low cost (b = 0.521 m), with around 95.2% desirability of its optimal solution. Overall, the current study represents a significant contribution and a vital step towards an accurate PRB's design based on previously determined optimal conditions.
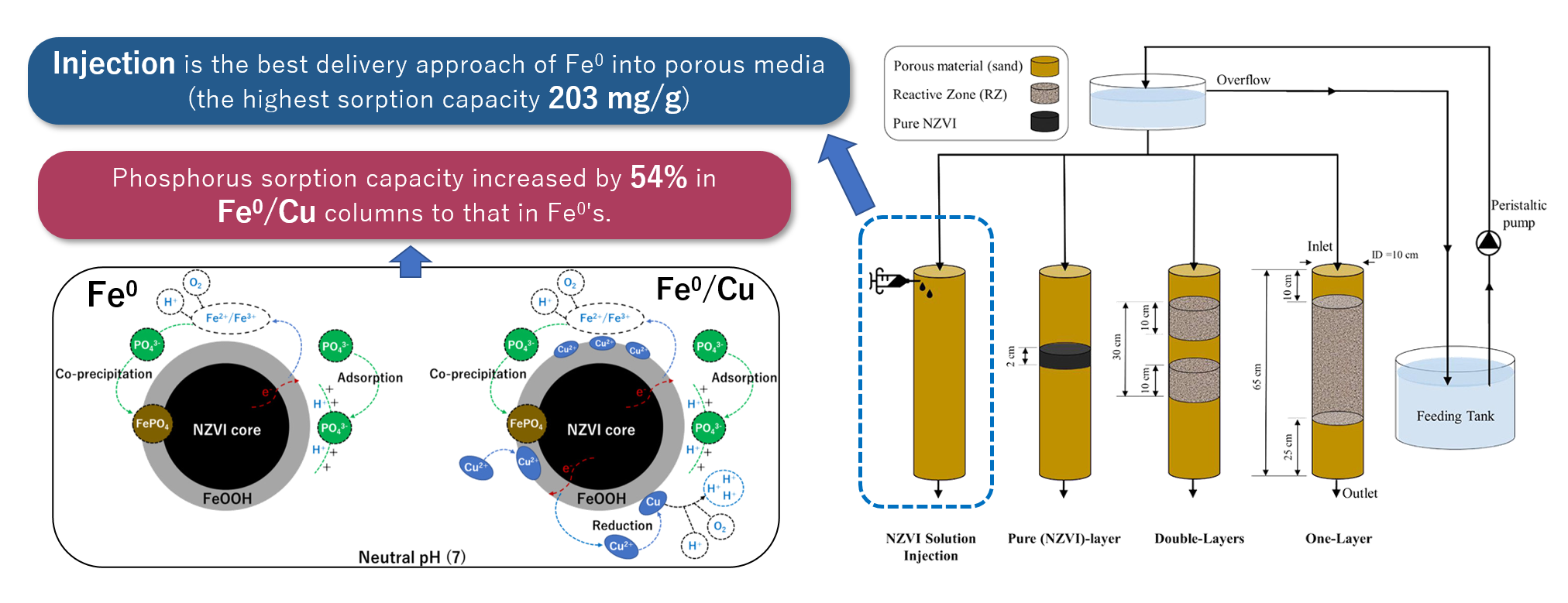
Investigating the Design Parameters for a Permeable Reactive Barrier Consisting of Nanoscale Zero-Valent Iron and Bimetallic Iron/Copper for Phosphate Removal
There is a growing interest in deploying nanoscale zero valent iron (NZVI) in permeable reactive barriers (PRBs) for groundwater remediation. In the present study a series of packed-column experiments were conducted in order to investigate the effectiveness of phosphorus removal from groundwater using NZVI and bimetallic NZVI/Cu as reactive materials within PRBs. Seven sets of packed-column experiments were conducted in order to study the effect of different design parameters for PRB; including delivery approach of NZVI into porous media, PRB's configuration, coexisting groundwater ions and change in flowrate. Results implied that doping NZVI surface with copper had an anti-aggregation effect and enhanced its performance in terms of phosphorus removal 2.2 times higher than bare NZVI. Moreover, the lower flowrate (10 ml/min) demonstrated improved phosphorus removal by 22% compared with higher flowrate (60 ml/min). Additionally, groundwater ions barely interfered phosphorus removal process with only ±6%. Overall, geochemical properties and characteristics of the supporting materials were key parameters in the removal process of phosphorus by NZVI/Cu.
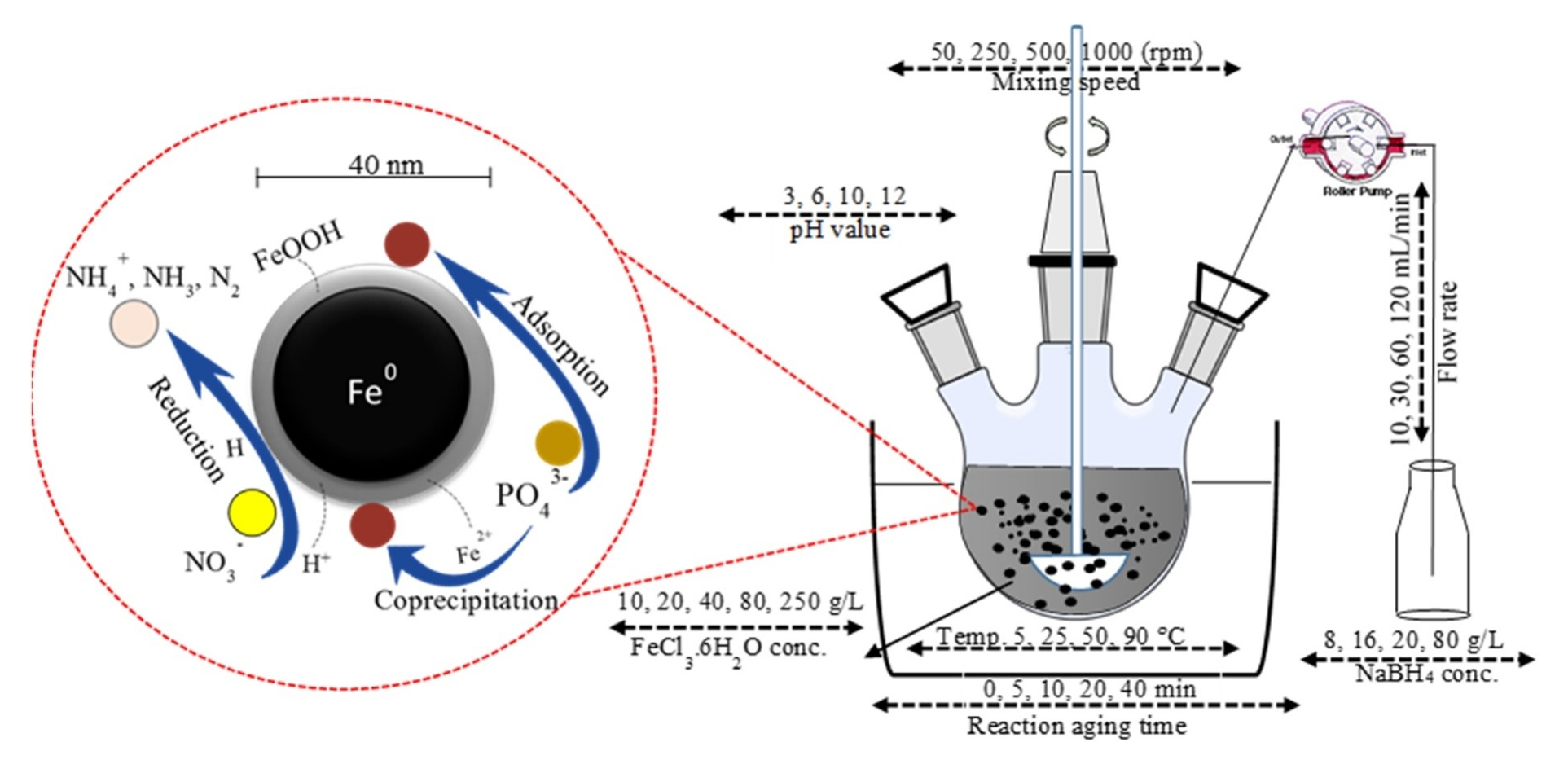
Improvement of Chemical Synthesis Efficiency of Nano-Scale Zero-Valent Iron Particles
This study investigated the synthesis conditions of nano-scale zero-valent iron (nZVI) formed by the chemical reduction method via an optimization process in order to enhance the nZVI’s reactivity. The properties of nZVI particles were characterized by transmission electron microscopy, laser diffraction particle size analyzer and X-ray diffraction. The performance of nZVI was evaluated towards the removal of nitrate and phosphorus from aqueous solutions. The optimization results for the effective variables, namely concentration, delivery rate and liquid volume of sodium borohydride (NaBH4), precursor concentration (FeCl3), reaction temperature, mixing speed, pH and aging time were demonstrated the improvement in nZVI reactivity. The results confirmed that nZVI proved high removal efficiency along with the lower particle size at NaBH4 concentration 16 g/L. The feeding rate of NaBH4 at 40 mL/min can effectively reduce the particle size and increase the nZVI reactivity. Increasing the NaBH4 liquid volume greatly improved the reactivity. Reduction of reaction aging time to 5 min and employing the acidic reaction medium of 6 pH greatly improved the nZVI reactivity. The highest mixing speed of 1000 rpm enhanced the phosphorus adsorption efficiency. However, high nitrate reduction was observed at 500 rpm. Increasing the reaction temperature resulted in decreasing the average particle size and the highest reactivity was achieved at 90°C. nZVI reactivity significantly was improved in the direction of low precursor concentration, and the highest efficiency was gained at 20 mg/L. In conclusion, the optimized parameters enhanced the overall efficiency of nZVI of nitrate reduction and phosphorus adsorption by 27% and 9.5% respectively.
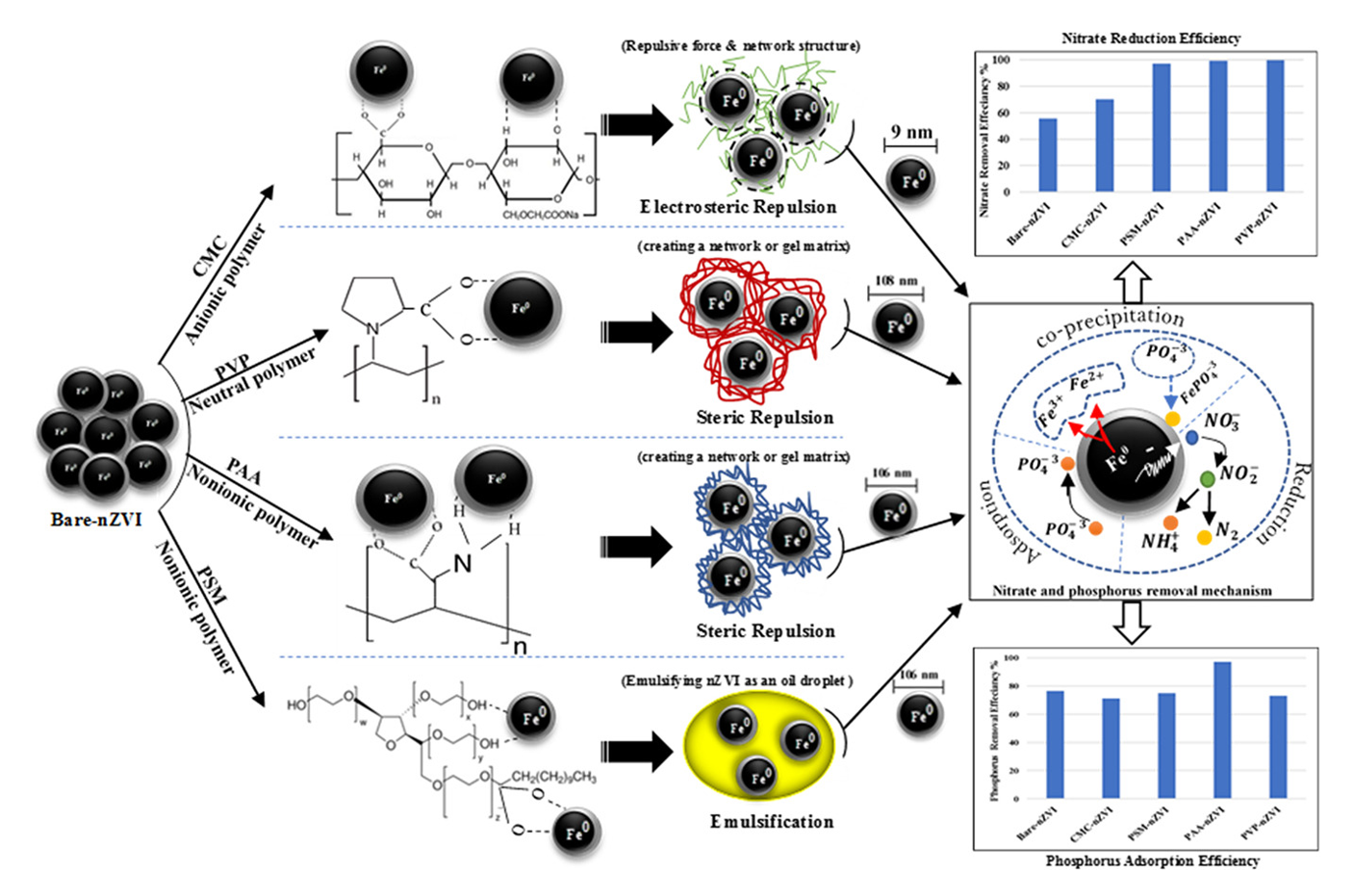
Enhancing the Characteristics and Reactivity of nZVI: Polymers Effect and Mechanisms
Nanoscale zero-valent iron (nZVI) is regarded as one of the most effective materials for environmental remediation. However, nZVI particles tend to aggregate rapidly due to their magnetic properties which leads to decrease their effectiveness in water treatment. To overcome the aggregation problem of nZVI particles and increase their reactivity, four different polymers were used during the synthesis of nZVI including polyacrylamide (PAA), carboxymethyl cellulose (CMC), Polyethylene sorbitan monolaurate (PSM) and polyvinylpyrrolidone (PVP). These polymers were used with different mass ratios varied between 0.04 and 0.40 %, in order to acquire the optimal mass ratio with nZVI and achieve the highest removal of nitrate and phosphorus. The mechanism of polymers adsorption onto the surface of nZVI was explored by conducting SEM-EDX, XRD, and FTIR analysis. TEM was used to examine the suface morphology of nZVI before and after being stabilized with 4 polymers. Results showed that, the sizes were found to be 9.53, 65.4, 106.4, 106.6 and 108.8 nm, using TEM and ImageJ, corresponding to CMC-nZVI, bare-nZVI, PAA-nZVI, PSM-nZVI and PVP-nZVI, respectively. The efficiency of bare and stabilized nZVI on nitrate reduction was found to be in the following the order: PVP-nZVI 99.5% > PAA-nZV 99% > PSM-nZV 97% > CMC-nZVI 70% > bare-nZVI 55.6%. Whereas, for phosphorus adsorption, PAA-nZV 97% was the most effective type, followed by bare-nZVI 76.3%, PSM-nZVI 75%, PVP-nZVI 73% and CMC-nZVI 71%. Therefore, PAA-nZVI exhibited an excellent performance over the rest for both nitrate and phosphorus removal at a wide range of pH.
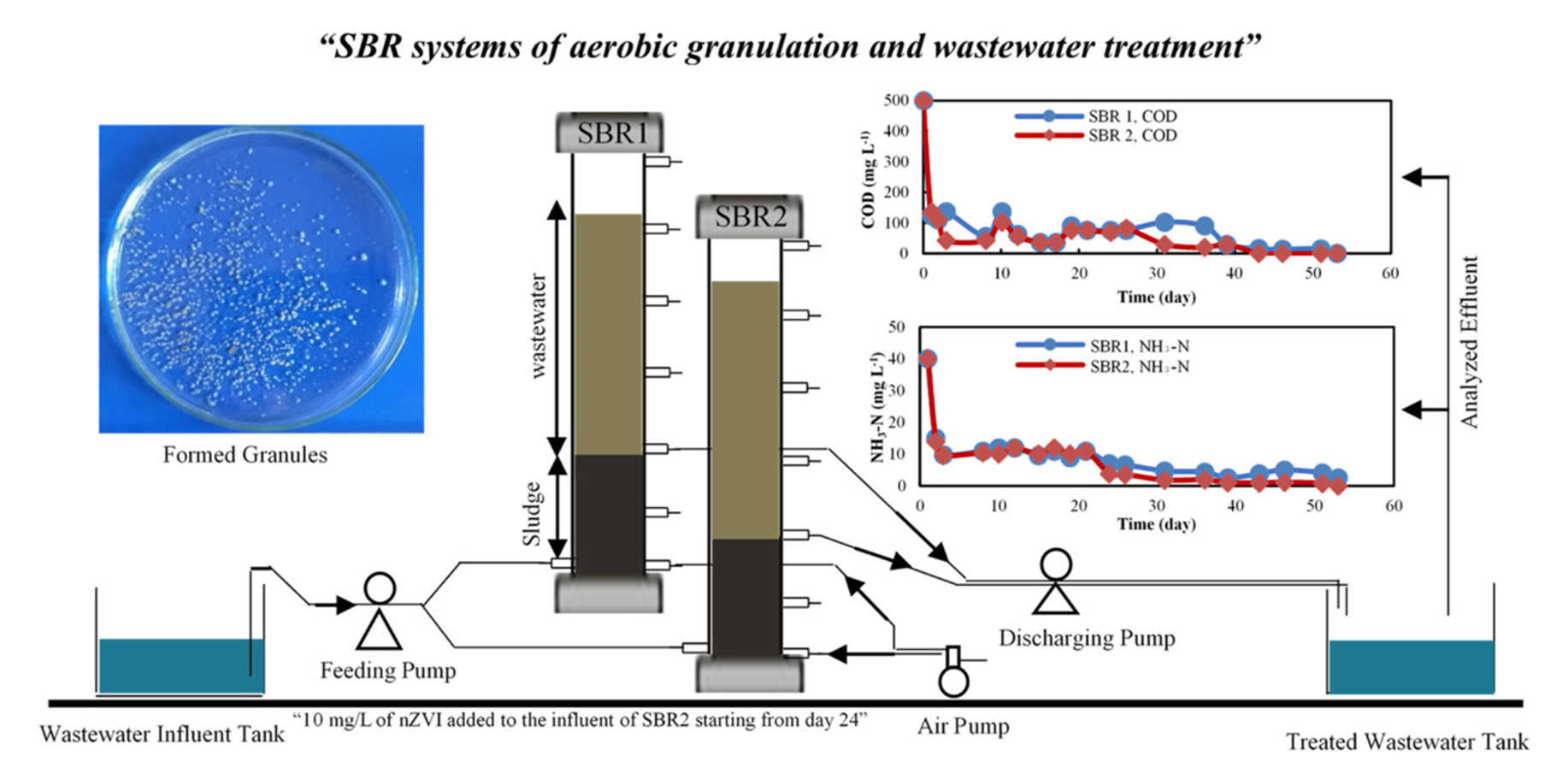
Impact of nZVI on the Formation of Aerobic Granules, Bacterial Growth and Nutrient Removal using Aerobic Sequencing Batch Reactor
The aim of this study was to investigate the effect of nanoscale zero-valent iron (nZVI) on the formation of aerobic granules, nutrient removal and bacterial growth during the treatment process of the municipal wastewater. For this purpose, two sequencing batch reactors (SBR) were simultaneously and automatically operated in a cyclic batch mode with four phases per cycle: feed, react, settle and decant. The sequential operation of the reactors consisted four cycles per day and lasted for sixty days in which 10 mg/L of nZVI particles were added to the influent of reactor 2. The reactors were fed with synthetic wastewater (3 liters per cycle) and acclimated with seed sludge collected from a full-scale municipal wastewater treatment plant in Istanbul. The effluent of the reactors was regularly analyzed for nitrate, nitrite, ammonia, phosphate and COD concentrations. In addition to that, their removal pathways including the direct adsorption to nZVI, utilization by microorganisms and adsorption within the generated granules were discussed. The removal efficiency of COD, ammonia and phosphate kept increasing, and almost a complete removal was observed after the formation of aerobic granules on day 50. Furthermore, after the addition of nZVI to SBR2 on day 24th, the removal efficiency of ammonia, COD and phosphate slightly improved. The addition of nZVI stimulated the production of Extracellular Polymeric Substances (EPS) in SBR2 including protein and carbohydrate generation. NGS analysis showed that the addition of nZVI into SBR2 increased the growth rate of some bacterial species such as Rhizobiales and Xanthomonadales and decreased others such as Clostridiales, confirming that the effect of nZVI on the bacterial growth was genera dependent. The aerobic granules were successfully formed in the reactors in less than 50 days and the addition of nZVI improved to some extent the size and settling rate of the formed granules in SBR2.